ELISA- Principle, Types and Applications
ELISA- Principle, Types and Applications
ELISA is an antigen antibody reaction. In 1971, ELISA was introduced by Peter Perlmann and Eva Engvall at Stockholm University in Sweden. It is a common laboratory technique which is usually used to measure the concentration of antibodies or antigens in blood.
ELISA is a plate based assay technique which is used for detecting and quantifying substances such as peptides, proteins, antibodies and hormones. An enzyme conjugated with an antibody reacts with colorless substrate to generate a colored product. Such substrate is called chromogenic substrate. A number of enzymes have been used for ELISA such as alkaline phosphatase, horse radish peroxidase and beta galactosidase. Specific substrate such as ortho-phenyldiamine dihydrochloride (for peroxidase), paranitrophenyl phosphate (for alkaline phosphatase) are used which are hydrolysed by above enzymes to give colored end product.
Principle
ELISAs are typically performed in 96-well polystyrene plates. The serum is incubated in a well, and each well contains a different serum. A positive control serum and a negative control serum would be included among the 96 samples being tested. Antibodies or antigens present in serum are captured by corresponding antigen or antibody coated on to the solid surface. After some time, the plate is washed to remove serum and unbound antibodies or antigens with a series of wash buffer. To detect the bound antibodies or antigens, a secondary antibodies that are attached to an enzyme such as peroxidase or alkaline phosphatase are added to each well. After an incubation period, the unbound secondary antibodies are washed off. When a suitable substrate is added, the enzyme reacts with it to produce a color. This color produced is measurable as a function or quantity of antigens or antibodies present in the given sample. The intensity of color/ optical density is measured at 450nm. The intensity of the color gives an indication of the amount of antigen or antibody.
Types of ELISA
Frequently there are 3 types of ELISA on the basis of binding structure between the Antibody and Antigen.
- Indirect ELISA
- Sandwich ELISA
- Competitive ELISA
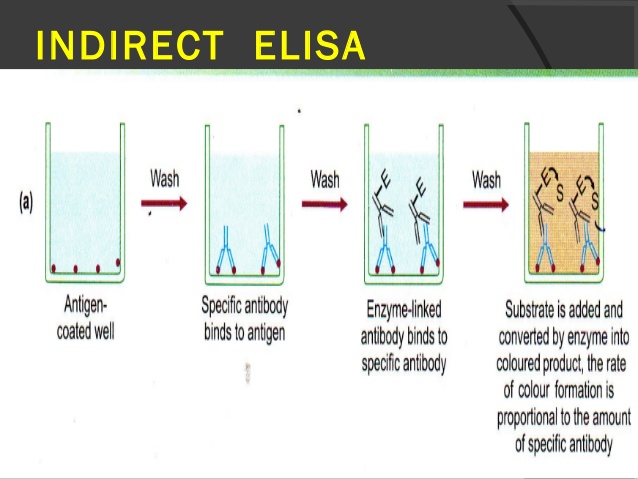
1. Indirect ELISA
Antibody can be detected or quantitatively determined by indirect ELISA. In this technique, antigen is coated on the microtiter well. Serum or some other sample containing primary antibody is added to the microtiter well and allowed to react with the coated antigen. Any free primary antibody is washed away and the bound antibody to the antigen is detected by adding an enzyme conjugated secondary antibody that binds to the primary antibody. Unbound secondary antibody is then washed away and a specific substrate for the enzyme is added. Enzyme hydrolyzes the substrate to form colored products. The amount of colored end product is measured by spectrophotometric plate readers that can measure the absorbance of all the wells of 96-well plate.
Procedure of Indirect ELISA
- Coat the micro titer plate wells with antigen.
- Block all unbound sites to prevent false positive results.
- Add sample containing antibody (e.g. rabbit monoclonal antibody) to the wells and incubate the plate at 37°c.
- Wash the plate, so that unbound antibody is removed.
- Add secondary antibody conjugated to an enzyme (e.g. anti- mouse IgG).
- Wash the plate, so that unbound enzyme-linked antibodies are removed.
- Add substrate which is converted by the enzyme to produce a colored product.
- Reaction of a substrate with the enzyme to produce a colored product.
Advantages
- Increased sensitivity, since more than one labeled antibody is bound per primary antibody.
- A wide variety of labeled secondary antibodies are available commercially.
- Maximum immunoreactivity of the primary antibody is retained because it is not labeled.
- Versatile because many primary antibodies can be made in one species and the same labeled secondary antibody can be used for detection.
- Flexibility, since different primary detection antibodies can be used with a single labeled secondary antibody.
- Cost savings, since fewer labeled antibodies are required.
- Different visualization markers can be used with the same primary antibody.
Disadvantages
- Cross-reactivity might occur with the secondary antibody, resulting in nonspecific signal.
- An extra incubation step is required in the procedure.
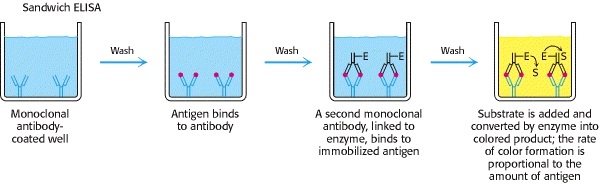
2. Sandwich ELISA
Antigen can be detected by sandwich ELISA. In this technique, antibody is coated on the microtiter well. A sample containing antigen is added to the well and allowed to react with the antibody attached to the well, forming antigen-antibody complex. After the well is washed, a second enzyme-linked antibody specific for a different epitope on the antigen is added and allowed to react with the bound antigen. Then after unbound secondary antibody is removed by washing. Finally substrate is added to the plate which is hydrolyzed by enzyme to form colored products.
Procedure of sandwich ELISA
- Prepare a surface to which a known quantity of antibody is bound.
- Add the antigen-containing sample to the plate and incubate the plate at 37°c.
- Wash the plate, so that unbound antigen is removed.
- Add the enzyme-linked antibodies which are also specific to the antigen and then incubate at 37°c.
- Wash the plate, so that unbound enzyme-linked antibodies are removed.
- Add substrate which is converted by the enzyme to produce a colored product.
- Reaction of a substrate with the enzyme to produce a colored product.
Advantages
- High specificity, since two antibodies are used the antigen is specifically captured and detected.
- Suitable for complex samples, since the antigen does not require purification prior to measurement.
- Flexibility and sensitivity, since both direct and indirect detection methods can be used.
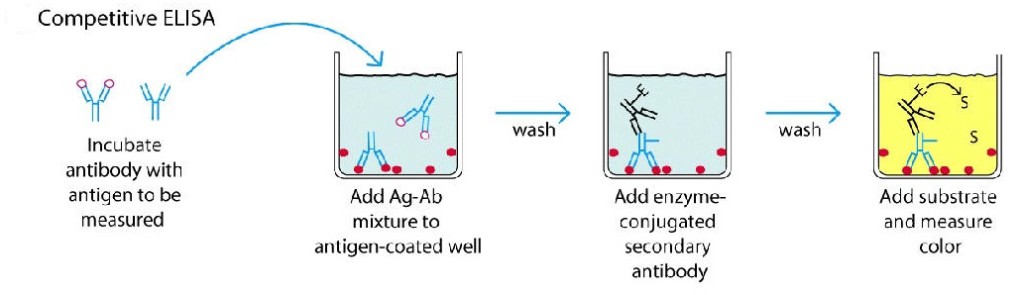
3. Competitive ELISA
This test is used to measure the concentration of an antigen in a sample.
In this test, antibody is first incubated in solution with a sample containing antigen. The antigen-antibody mixture is then added to the microtitre well which is coated with antigen. The more the antigen present in the sample, the less free antibody will be available to bind to the antigen-coated well. After the well is washed, enzyme conjugated secondary antibody specific for isotype of the primary antibody is added to determine the amount of primary antibody bound to the well. The higher the concentration of antigen in the sample, the lower the absorbance.
Procedure
- Antibody is incubated with sample containing antigen.
- Antigen-antibody complex are added to the microtitre well which are pre-coated with the antigen.
- Wash the plate to remove unbound antibody.
- Enzyme linked secondary antibody which is specific to the primary antibody is added.
- Wash the plate, so that unbound enzyme-linked antibodies are removed.
- Add substrate which is converted by the enzyme into a fluorescent signal.
Advantages
- High specificity, since two antibodies are used.
- High sensitivity, since both direct and indirect detection methods can be used.
- Suitable for complex samples, since the antigen does not require purification prior to measurement.
Application of ELISA
- Presence of antigen or the presence of antibody in a sample can be evaluated.
- Determination of serum antibody concentrations in a virus test.
- Used in food industry when detecting potential food allergens.
- Applied in disease outbreaks- tracking the spread of disease e.g. HIV, bird flu, common, colds, cholera, STD etc.

cassate elisa
I love this site because it helps in my assignments
Is ELISA use to diagnose every disease? Does it include in complete blood test ?
Is ELISA a screening test?
GOOD NOTES IN SIMPLE LANGUAGE.
Well imperative note sir.thnks
Good notes..very simple language…
Good concept, I like it
Really good..:)
CAN I USE ELISA KIT TO QUANIFY AFLATOXINS IN ANIMAL FEED
yes
In my view, this is very good note especially for those students who are studying in medical as well as veterinary medical streams and also will be useful material for lab technicians.
I agree sir .very simple n clear notes.easy to understand
I appreciate, simple to understand
well explained and thanks
Very nice notes I really loved it
Good.. Love it
V good material.Being a microbiologist i never ever read such discriptive and in simple wording notes.excellent
Really easy to understand
Wow this ar gud explaination, thank u so much
It is a very good site, very well explained & easy to understand the basics… Thanks a lot.i really really loved i.
BEAUTIFULLY EXPLAINED
HELPED ME A LOT
THANK U SIR
The text made it easier to understand the principle behind the ELISA and yes ts pricise.
Well notes!
nice notes in easy language …👍
very easy language . help me a lot inpreparing my presentation about ELISA
I like it very much,its a simple and clear explanation.
Thanx u helped me alot
exact and good explanation..which helped me a lot for quick reference….
Thanks Sir. This well explained in simple terms and diagrams. It has helped me to really understand ELISA assay methods. Now I can defend my thesis.
Thank you so much!
Thank you so much.it is help me a lot….
best notes for learning
Good site helps in assignment
thanks…. this is very helpful me
Very informative in simply explained sir thanks
Very informative notes and simply explained thanks sir
Its very helpfull. Its simplifies and summarised the whole idea about ELISA assays. Thank you..
GOOD NOTES,THANKS,,..
Well sir nice notes simple language
ELISA test is helpful for the detection of which ?
make sense.. lovely
It’s very useful
Thank you
Keep doing.
Thanks alot for sharing this note
Good and easy notes to help one do assignment…Thank you.
Good one
Wow ,easy understanding very helpful for my examination
Good notes very helpful for me
nice notes very helpfull to all med. students simple way of explanation very nice
well summerized notes and easy to memorize. Thanks
Very informative notes
Good and precise. I like it