Bile Solubility Test- Principle, Procedure, Result Interpretation, Examples and Limitation
Bile Solubility Test- Principle, Procedure, Result Interpretation, Examples and Limitation
This test is used to differentiate Streptococcus pneumoniae, which is soluble in bile and bile salts, from alpha- hemolytic Streptococcus which are insoluble.
Principle
The bile solubility test is based on the observation that Streptococcus pneumoniae visibly lyse when 2% or 10% sodium desoxycholate (bile salts) is applied to the colony under specific condition of time and temperature, but other Streptococci do not lyse. Lysis depends on the presence of an intracellular autolytic enzyme, an amidase, which is produced by Streptococcus pneumonia and is activated by bile salts. Bile salts lower the surface tension of the medium-membrane interface and under these condition, Streptococcus pneumonia is susceptible to disruption by enzyme action thus enhancing the organism’s natural autolysis process.
The working mechanism of the test is not clearly understood; however one theory is that the bile salts facilitate lysis of pneumococcal cells by activating the autolytic enzyme.
Procedure
Bile salt preparation
- Prepare 10% bile salt solution for plate method: Dissolve 10 gram of sodium desoxycholate in 100 ml distilled water.
- Prepare 2% bile salt solution for test tube method. Dissolve 2 gram of sodium desoxycholate in 100 ml distilled water.
NOTE: Store at 15-30°C. Storage of the reagent at cool temperature can cause it to thicken.
A. Test tube Method
- Prepare a suspension of the organism by adding few colonies from freshly isolated bacterial growth (18-24 hour) in a test tube containing 1-2 ml of sterile saline.
- Shake or vortex to form a uniform suspension. Adjust the suspension to that of 0.5-1 McFarland standard.
- Divide the organism suspension equally into two tubes, one labeled TEST and other labeled CONTROL.
- Dispense 2 drops of 2% sodium desoxycholate into the tube labelled TEST. Add 2 drops of saline to the labelled CONTROL.
- Gently mix each tube.
- Incubate the tubes at 35-37°C.
- Observe the tubes for clearing of turbidity after 10-15 minutes. Examine each tube by Gram-staining or Methylene blue wet mount for lysis of the cell. If negative, continue to incubate up to 3 hours. Observe again for clearing.
B. Direct plate method
- Place a drop 10% sodium desoxycholate to the side of a young well-isolated colony (18-24hrs) growing on 5% sheep blood agar.
- Gently roll the drop over the colony by tilting the plate, without dislodging the colony from the agar.
(NOTE: Do not touch the agar surface with the tip of the dropper of bile reagent).
- Incubate the plate at 35-37°C for 15-30 minutes or until the drop has evaporated.
- Examine for lysis of colony.
C. Direct slide blood culture method
- Add 1 drop of blood culture broth to 1 drop of bile salt on a glass slide and allow to dry.
- As control, add 1 drop of broth blood culture to 1 drop of water and allow to dry.
- Gram stain and examine for cocci.
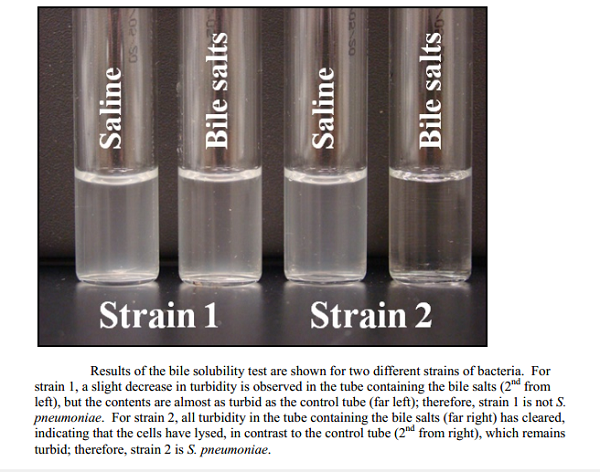
Result Interpretation
A. Test tube method
Positive: Clearing of the turbidity in test suspension; control suspension remain turbid.
Negative: No clearing of turbidity in test and control suspension.
B. Direct plate method
Positive: Colony disintegration or flattening of the colony within 30 minutes, leaving an alpha-hemolytic where colony may be located.
Negative: Intact colony or no change in the integrity of the colony within 30 min.
C. Direct slide blood culture method
Positive: No cocci are seen in the test smear and control smear shows intact bacteria.
Negative: cocci are seen in both test smear and control smear.
Examples
Positive: Streptococcus pneumoniae
Negative: Enterococcus faecalis
Quality control
- Test new lot of reagent with known positive and negative controls before putting it into use.
- Do not use if bile reagent is not clear and very light amber.
- Organisms:
Positive: Streptococcus pneumoniae ATCC 49619
Streptococcus pneumoniae ATCC 6305
Negative: Enterococcus faecalis ATCC 29212
Streptococcus sanguis ATCC 10556
Limitation
- Bile solubility is used only to differentiate pneumoniae from other alpha-hemolytic streptococci.
- Some pneumoniae organisms will not lyse in the presence of bile, possibly due to the loss of virulence factor or capsule. If lysis is not present, the isolate may still be S. pneumonia. Therefore, colonies resembling S. pneumoniae which are not bile soluble should be further identified using another method, such as optochin susceptibility and/or DNA probe.
- Partial clearing is not considered positive for pneumococcal identification. Partially soluble strains that have optochin zones of inhibition of less than 14mm are not considered pneumococci.
- Normal autolysis of pneumoniae may be inhibited by a high concentration of bile salts. Evaporation may cause the reagent to become more concentrated, thus affecting the test.
- Bile solubility test is not reliable with old cultures since they have lost their active enzyme, resulting in a false-negative test.
- When performing the bile solubility tube test using saline or unbuffered broth, it is essential to adjust the pH to neutral before adding the reagent, in order to avoid false-negative reactions.
- When testing using the plate method, care must be taken not to dislodge the colony being tested, thus leading to false-positive results. If the direct plate is difficult to interpret, the test should be repeated using the tube or slide method.