CAMP Test- Principle, Purpose, Procedure, Result and Limitation
CAMP Test- Principle, Purpose, Procedure, Result and Limitation
CAMP test was first identified by Christie, Atkins, and Munch-Peterson in 1944 and CAMP test is an acronym of three researchers.
CAMP (Christie, Atkins, and Munch-Peterson) test is used for the presumptive identification of Group B Streptococci (Streptococcus agalactiae). It is the only beta-hemolytic Streptococcus which secrete a protein called CAMP factor or “protein B”. CAMP test rarely give false positive with other Streptococci.
Variety of methods are currently available to identify Group B Streptococci (GBS) isolated from clinical specimens. The standard CAMP test and the CAMP spot test (rapid test) are mostly used. Standard camp test are time consuming and/or expensive compared to the CAMP spot test.
Principle
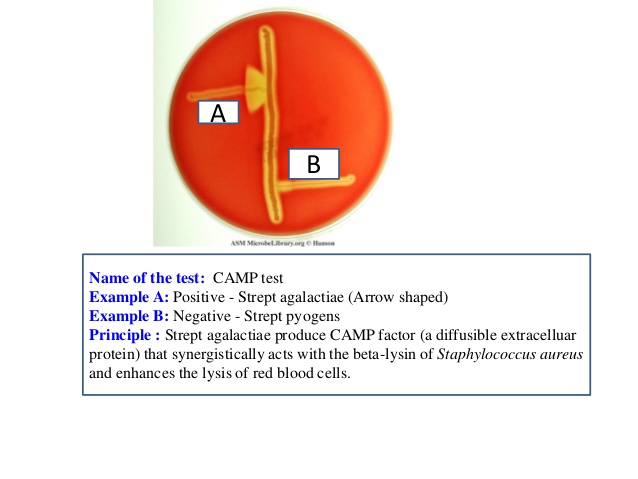
CAMP test detects the production of diffusible, thermostable, extracellular protein known as CAMP factor, produced by Group B Streptococcus. The CAMP factor acts synergistically with the beta lysin produced by Staphylococcus aureus to produce a zone of enhanced lysis of sheep or bovine erythrocytes. The standard CAMP test depend on the elaboration of two toxins during growth to form a typical arrowhead or flame-shaped clearing at the junction of the two organisms when they are placed perpendicular to each other.
The rapid test utilizes an extract of Staphylococcal beta-lysin that acts directly with the CAMP factor previously diffused in the medium around the S. agalactiae colony. A positive CAMP reaction is indicated by an enhanced hemolysis within 30 minutes to 1 hour of adding a drop of CAMP factor reagent.
Purpose of CAMP test
This test is useful in the identification of both S. agalactiae and many gram-positive rods, including Listeria monocytogenes.
Procedure of CAMP Test
A. Standard CAMP test
- Using an inoculating loop, streak a beta-lysin-producing Staphylococcus aureus (ATCC25923) in a straight line across the center of a sheep blood agar plate.
- Streak test organism in a straight line perpendicular to the S. aureus leaving 1cm space between the two streaks. (Multiple organisms can be tested on a single plate if they are 3 to 4mm apart).
- Incubate the plate at 37 degree Celsius in ambient air for 18-24 hours.
B. CAMP Spot test
- Remove test kit from the freezer and allow it to thaw.
- Using a pipette, place one drop of CAMP spot test reagent next to a characteristic colony grown for 18-24 hours on blood agar plate.
- Incubate the plate aerobically or in 5-10% CO2 at 37°C for 20-30 minutes.
- Observe, using transmitted light, for an arc or circle of enhanced hemolysis next to the colony. If reaction is negative, re-incubate for an additional 30 minutes.
Result Interpretation of CAMP Test
Positive: Enhanced hemolysis is indicated by an arrow head-shaped zone of beta-hemolysis at the junction of the two organisms.
In CAMP Spot test positive result is indicated by the presence of clear zone (arc or circle) of enhanced hemolysis.
Negative: No enhancement of hemolysis.
In CAMP Spot test negative result shows no area of enhanced hemolysis near the colony in the presence of test reagent.
NOTE: A similar test has been described for Listeria ivanovii, where an “arrowhead” hemolysis occurs appear between streaks of Listeria ivanovii and Rhodococcus equi.
Quality control
Positive: Streptococcus agalactiae ATCC 13813
Negative: Streptococcus pyogenes ATCC19615
CAMP Test for the identification of Listeria monocytogenes
A version of the CAMP test was first used by Groves to identify Listeria monocytogenes. He found that the pathogenic Listeria monocytogenes was also positive for CAMP test. Listeria monocytogenes is a biosafety level 2 organism and should be handled with precautions.
To perform CAMP test for the identification of Listeria monocytogenes, Listeria monocytogenes is streaked at right angle to the streak of beta-hemolytic Staphylococcus aureus on sheep blood agar plate. Then incubate the palte in ambient air at 37 degree Celsius for 24 hours.
Positive CAMP: Enhanced zone of beta-hemolysis and a smaller less obvious rectangular zone of hemolysis.
Limitation of CAMP test
- Some Group A Streptococcal will be CAMP test positive if the plate is incubated in a candle jar in an atmosphere or under anaerobic conditions. Therefore, ambient air incubation should be done.
- Extended incubation times or elevated incubation temperatures may give false-positive results.
- Sheep blood agar plates are only used. Human, horse, rabbit, or guinea pig blood plates will not give a proper reaction.
- L. ivanovii only shows a positive CAMP reaction when using an alternative CAMP test method, in which Rhodococcus equi replaces S. aureus.
- Colonies of Listeria monocytogenes have a narrow zone of beta-hemolysis on sheep blood agar and may be confused with group B beta-hemolytic streptococci, if catalase and gram stain are not performed.


I love it
Very nyc explanations bravo!
Very nice explanations
very nicely represented.. cleared my concepts. love it.
Like it