HAEMAGGLUTINATION ASSAY – VIRAL QUANTITATION
HAEMAGGLUTINATION ASSAY – VIRAL QUANTITATION
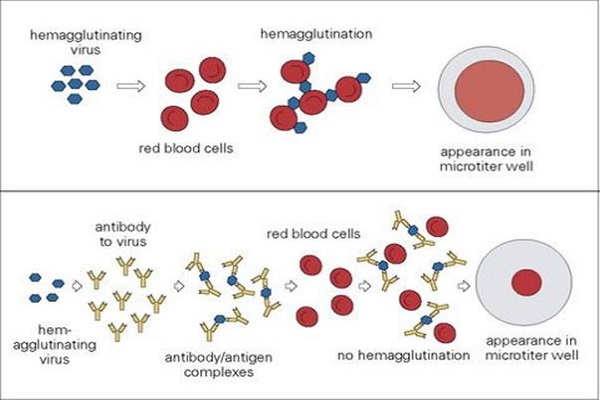
Haemagglutination assay was developed by American virologist George Hirst in 1941-1942. The ability of certain viruses to bind with the red blood cells through their superficial glycoproteins and proteins had been utilised to quantitate these viruses and the assay is termed as haemagglutination assay. Red blood cells when suspended with adequate amount of such viral load is capable of forming lattices coating the container. But with insufficient viral load, red blood cells form sharp dots at the centre of the container indicating absence of agglutination.
Considering these facts, the unknown samples are diluted and analysed for the last dilution point, also called end point, capable of agglutinating the red cells completely. The amount of virus in the end point is termed as one hemagglutination unit (1 HAU). Considering the following protocol, 1 HAU is the amount of virus in 50 µl volume of sample required to agglutinate 50 µl of the 0.5% chicken RBC. However, the amount of virus may be different in 1 HAU depending upon the scaling of the process. For example in macroscale process done in test tubes involving higher amount of sample and RBCs, the amount of virus in 1 HAU will definitely rise significantly. Additionally, the reciprocal of the dilution of the virus at end point is said to be the haemagglutination titre (HA titre).
AIM
The main purpose of the assay is to estimate HA titre for the unknown viral sample. The basis of this assay is the ability of viral haemaglutinin to bind with the sialic acid present on the receptors of surface of the red blood cells causing haemagglutination.
MATERIALS
Dilution tube, micropipette (40-200 µl) and tips, V-bottom plate (96 well), Phosphate buffer saline (20 ml, pH – 7.2), 1:10 pre-diluted A/PR/8/34 virus and 0.5 % chicken red blood cells.
METHODS
- Label the first row of 96-well V-bottom plate as 1-12.
- Add 50 µl of the PBS buffer from well 2 till well 12.
- Add 100 µl of the pre-diluted virus in well 1.
- Perform serial dilution of the viral sample from well 1 to 11 using 50 µl pipette.
- Discard 50 µl of the diluted solution from well 11 to make the volume of the solution even in all the wells (which is required for getting the right amount of virus in the well).
- Then, add 50 µl of properly mixed 0.5% chicken red blood cells to each well (1-12).
- Incubate for approximately 30 minutes at room temperature (to ensure the occurrence of haemagglutination).
- Lastly, note the end point of the sample and observe the pattern of haemagglutination to calculate HA titre.
SAMPLE RESULT
Table: Haemagglutination pattern of serially diluted influenza virus for determination of HA titre
- Lattice formation upto well 6 shows the presence of one hemagglutination unit (1 HAU) viral load in the well.
- HA Titre is the reciprocal of the highest dilution upto which haemagglutination was observed.
Here,
HA titre = 320
- Absence of hemagglutination from well 7 to well 11 – showing no or insufficient viral load to cause agglutination.
- Well 12: Test control
IMPORTANT NOTES
This haemagglutination assay is found to be effective, simple and easier to understand as compared to the other robust techniques, like nucleic acid amplification tests, used to assess the virus qualitatively as well as quantitatively. However, from this assay it is difficult to predict the subtypes of virus. Furthermore, if an unknown sample is to be analysed, this assay is not suitable for unravelling riddles of diagnostic approach because of the binding of red blood cells to various viruses or viral proteins. For these reasons, it is more likely to be a screening test rather than diagnostic assay. But the viral quantitation for a known type can be performed using this assay. Despite the simpler handling, high sensitivity, lower chances of error during performance, minimal skill requirement, relatively low cost and rapid assessment, this assay has very low specificity. Meanwhile, slight modification of the assay to haemagglutination inhibition assay, which detects specific antibodies against viral antigens, is much more specific that may be helpful in determining the quality of the sample effectively. In addition to that, coating erythrocytes with specific antibodies can be beneficial in increasing the specificity of the assay. Thus, evidences support the modification of haemagglutination to be useful for ascertaining both quality and quantity of viruses.