Quellung Reaction For Pneumococci
Quellung Reaction For Pneumococci
Streptococcus pneumoniae (Pneumococcus), a normal inhabitant of the human upper respiratory tract, is one of the major causative agents of bacterial pneumonia, meningitis, sepsis, bacteremia and otitis media.
The traditional methods for identification of S. pneumoniae are presumptive procedures based on Gram stain reaction, alpha-hemolysis, optochin susceptibility, and bile solubility. Identification of streptococci directly from blood cultures has been evaluated. Tentatively identified pneumococcal isolates can be confirmed by serological methods using Neufeld’s Quellung reaction with polyvalent antiserum. The latex agglutination procedure permits a simple and more rapid serological identification of Streptococcus pneumoniae isolates for the clinical laboratory.
The gold standard method for pneumococcal typing is the capsular reaction test, known as the Quellung reaction, first described by Neufeld in 1902. This technique utilizes a microscope and specific pneumococcal antisera and is commonly used in reference and research laboratories worldwide.
Principle
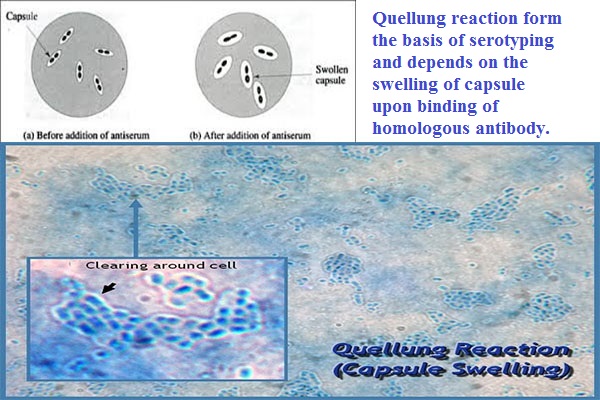
As a rapid method to definitively identify Streptococcus pneumoniae directly in specimen, capsular swelling of the pneumococcus in the presence of specific capsular antibody is observed.
The capsular reaction is a result of the interaction between pneumococcal capsular polysaccharide and its homologous antibody. Antigen- antibody reaction between antiserum and the capsule causes the capsule to appear to swell; although the mechanism is probably due to a change in the refractive index of the capsule that enhances its visibility. After the addition of a counter stain (methylene blue), the pneumococcal cells stain dark blue and are surrounded by a sharply demarcated halo which represents the outer edge of the capsule. The light transmitted through the capsule appears brighter than either the pneumococcal cell or the background.
Significance
This test can be used to identify difficult isolates of presumptive pneumococci that are bile insoluble or optochin negative or to type these organisms after they have already been isolated and identified, using specific capsular typing sera, for evaluation of vaccine efficacy.
Procedure
- Divide a slide into two section. Label one section as the test and other as the control.
- Grow the isolates to be tested for 18-24 hours on a BAP at 35-37°C with ~5% CO2 (or in a candle-jar).
- A small amount of bacterial culture from overnight growth on BAP is transferred and mixed into a droplet(0.5ml) of 0.85% phosphate buffered saline (approximately make equal to 0.5 McFarland density standard).
- Place a drop of specimen (0.2-1µl) suspension onto each of two section of a slide, spread out and allow to air dry.
- Place a small drop (5 µl) of antiserum on the first sections of a slide and spread out over the specimen.
- Place equal amount of rabbit serum on the second section of the slide.
- Put equal amount (5 µl) of methylene blue solution on each of two cover-slips. Invert the cover-slips onto each section of the slide.
- Incubate at room temperature for 10-15 minutes.
- After 10 minute, read the test and control slides under microscope using an oil immersion lens.
Result interpretation
Positive: Swelling of the capsules in comparison to the control, giving a sharply demarcated halo. Capsules may be visible in the control but do not produce a clear demarcation and glassy appearance.
Negative: No appearance of a clear, enlarged halo surrounding the stained cell.
No difference between the test and control cells.
Limitation
- Negative test does not indicate that the organism is not S. pneumoniae, since the organism could have lost the ability to express a capsule.
- The test can be falsely negative if there are too many organisms on the slide (antigen excess), if this occurs, the test should be repeated with fewer organism.
- If culture identification is in doubt, additional tests should be performed i.e. optochin susceptibility, bile solubility test, and/or biochemical testing for gram positive cocci.
- False-negative reactions may occur in the 4-hour broth test due to insufficient growth. In such cases, the colony test should be performed using a fresh 18 hr subculture.

No comment